The term standard state is used to describe a reference state for substances and is a help in thermodynamical calculations as enthalpy entropy and gibbs free energy calculations.
Vinyl chloride standard enthalpy of formation.
Gas c 2 h 2.
The superscript degree symbol indicates that substances are in their standard states.
The major industrial preparation of vinyl chloride begins with ethylene and has two.
Vinyl chloride a colourless flammable toxic gas belonging to the family of organohalogen compounds and used principally in making polyvinyl chloride or pvc a widely used plastic with numerous applications.
Enthalpy of formation of gas at standard conditions data from nist standard reference database 69.
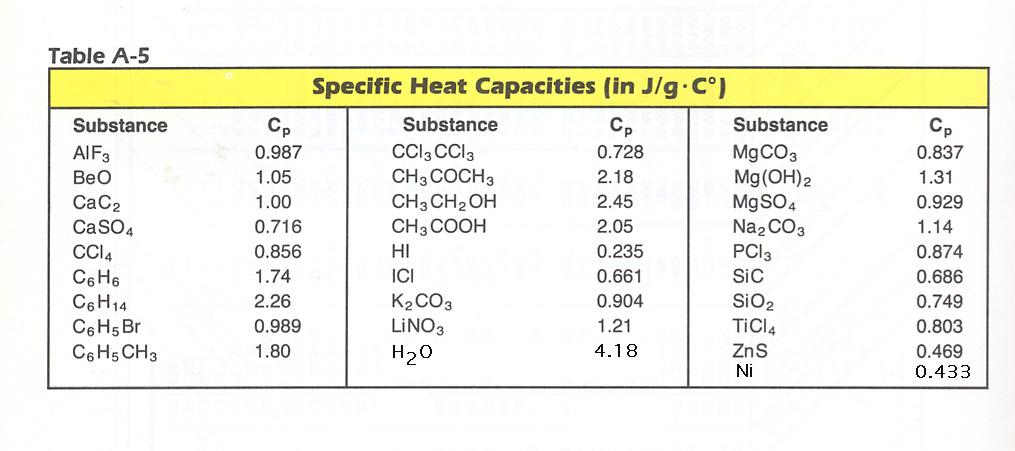
Solid alcl 3 705 63 aluminium oxide.
δh δg s definitions of standard states.
About 13 billion kilograms are produced annually.
And was also used for the initial development of high accuracy anln composite electronic structure methods.
The heat association with dissolving a particular solute in a particular solvent.
Nist chemistry webbook the national institute of standards and technology nist uses its best efforts to deliver a high quality copy of the database and to verify that the data contained therein have been selected on the basis of sound scientific.
Vinyl chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition.
Also called standard heat of formation enthalpy of solution.
208 53 j mol k enthalpy of combustion δ c h o liquid 1236 4 kj mol heat capacity c p.
Ns shuman ma ochieng b sztaray t baer tpepico spectroscopy of vinyl chloride and vinyl iodide.
The change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states.
Solid c 2 h 3 cl 94 12 ethyne.
1 2 enthalpy of formation based on version 1 118 of the thermochemical network this version of atct results was partially described in ruscic et al.
Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics.
A 2008 112 5647 5652.
Standard enthalpy change of formation data table author.
Neutral and ionic heats of formation and bond energies j.
J mol k heat capacity.
Solid al 2 o 3 1669 8.
129 0 j mol k gas properties std enthalpy change of formation δ f h o gas 125 4 kj mol standard molar entropy s o gas.
Standard enthalpy of formation.