Vinyl esters have enhanced mechanical properties compared to polyesters with physical strength better impact and thermal shock resistance.
Vinyl ester molecular structure.
Vinyl acetate is a commercially important monomer that is classified as a vinyl ester i e.
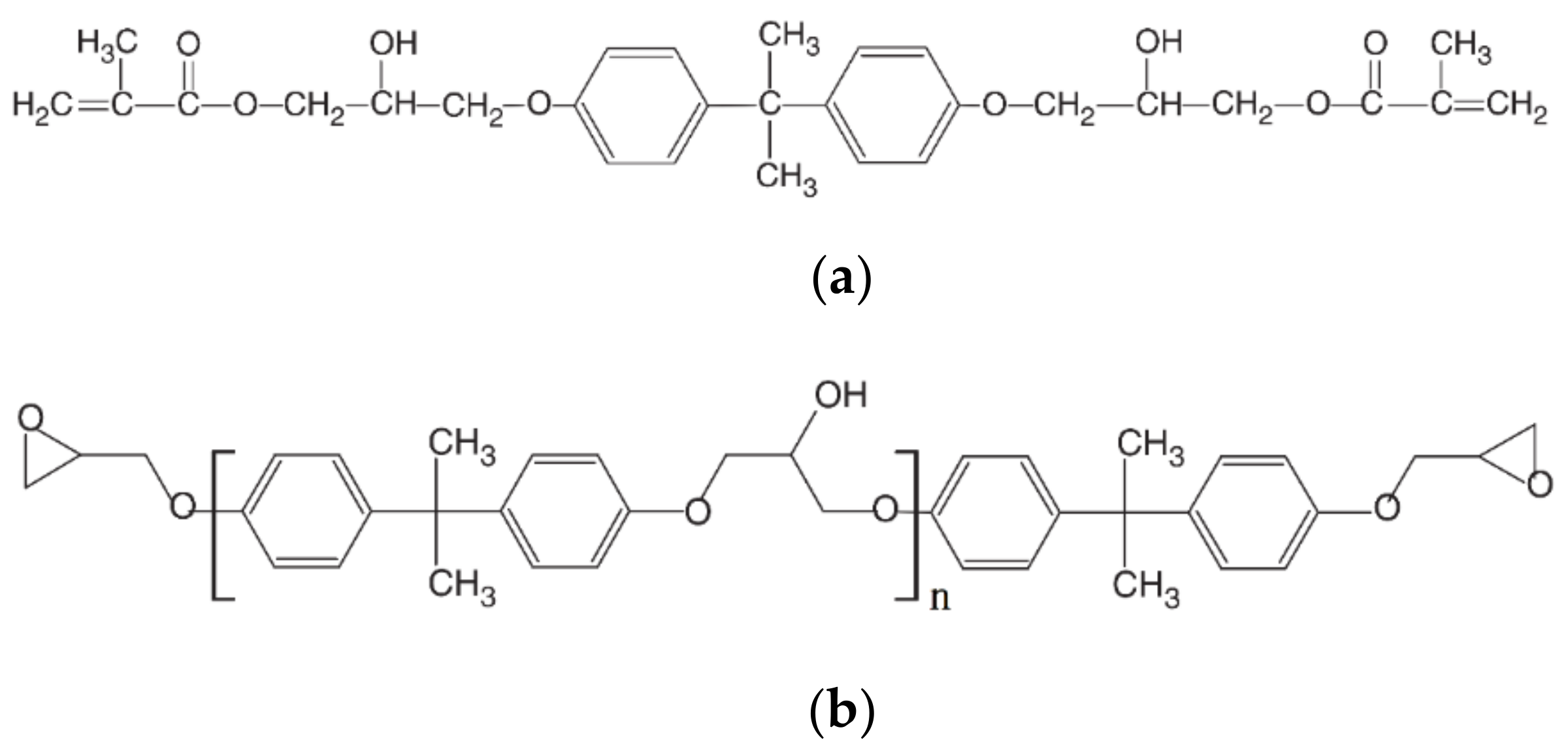
The molecular structure of vinyl ester resins is similar to that of polyesters but differs primarily on the location of their reactive groups which are positioned only at the ends of the chains.
This makes it much more resistant to water penetration hydrolysis which can cause osmotic blistering.
Vinyl ester monomer contains two vinyl end groups that allow cross linked structure to form during the reaction.
2 acetoxybenzoic acid vinyl ester.
As the length of the chain is available to absorb impact loads this makes vinyl ester resins more durable and resilient than polyesters.
Chemical structure of the vinyl ester resin monomer.
Ethylene is now the preferred feedstock for vam vinyl acetate monomer largely replacing the earlier acetylene based process.
Vinyl esters undergo homopolymerization via a radical mechanism.
Vam is usually produced by the catalysed vapour phase reaction of acetic acid with ethylene and oxygen in a fixed bed tubular reactor using a supported noble metal catalyst.
Commercially important examples of these monomers are vinyl acetate vinyl propionate and vinyl laurate.
The most widely used member of the category is vinyl acetate.
Vinyl esters shrink less on curing which means that pre release of a laminate from a mold is less significant.
Vinyl ester refers to esters formerly derived from vinyl alcohol.
Vinyl esters are more tolerant of stretching than polyesters.
Vinyl ester or vinylester is a resin produced by the esterification of epoxy resin with unsaturated monocarboxylic acid.
An ester of vinyl alcohol.
Vinyl acetate is not as hydrolytically stable in polymers as are the structurally similar acrylic esters.
Vinyl ester has fewer open sites in its molecular chain.
2 acetoxy benzoic acid vinyl ester.