Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed expired fee related application number us07 507 349 inventor walter r.
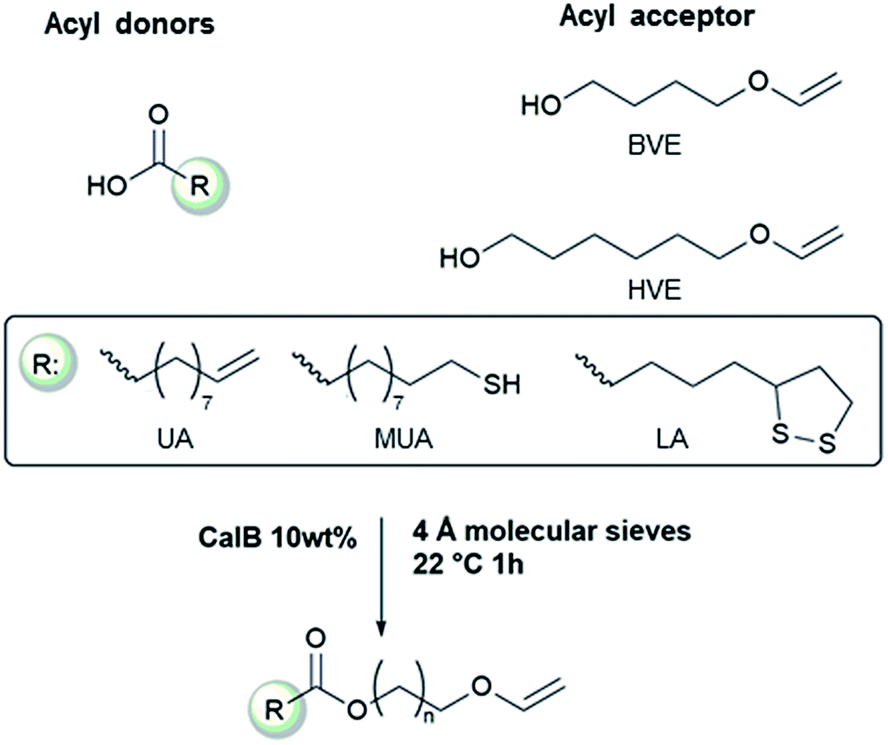
Vinyl ether carboxylic acid.
Table 1 shows the properties of polycarboxylic acids before and after blocking.
Vinyl allylic aryl benzylic n h h o h o oh nh2 o sh ch3 o oh h2 n oh oh o h o oh2 above 50 7 2 to 3 2 to 3 4 5 9 10 10 10 16 18 18 20 25.
The reaction of vinyl ethers with carboxylic acids using iodine as a catalyst under solvent free conditions was investigated.
Acid vinyl ether poly carboxylic acid prior art date 1990 04 10 legal status the legal status is an assumption and is not a legal conclusion.
The other carboxylic acids and vinyl ethers were commercially avail able and they were used as received.
Mechanistic studies revealed the production of the adduct of the vinyl ether with.
In the present study vinyl ether alcohols and functional carboxylic acids were used to synthesize bifunctional vinyl ether esters using the immobilized enzyme candida antarctica lipase b as a catalyst.
The facile general acid catalyzed conversion of 2 ethoxy 1 cyclopentene 1 carboxylic acid to cyclopentanone.
The alkene also undergoes diels alder and 2 2 cycloadditions.
2 3 esteri cation of 3 hydroxy acids with vinyl ether the typical procedure is as follow s entry 11 in table 5.
Protonated alcohol or ether pka 2 to 3 h2 35 3.
Most of blocked carboxylic acids obtained were liquid.
Acetic acid adds in the presence of palladium catalysts to give ethylidene diacetate ch 3 ch oac 2.
22 23 esterification of bis mpa with 2 chloroethyl vinyl ether followed by cyclization using ethyl chloroformate yielded cyclic carbonate monomer.
Carboxylic acids without the need for derivatives or scavengers would be preferred for the synthesis of vinyl ether functional esters.
It undergoes transesterification with a variety of carboxylic acids.
Tan 2 yl acetic acid 12 were prepared from the corre sponding ketones as previously reported 1 5.
A vinyl ether functionalized cyclic carbonate monomer 5 methyl 5 2 vinyloxy ethyl oxycarbonyl 1 3 dioxan 2 one mvec 2 was synthesized by a well established two step procedure from bis mpa.
Vinyl ethers are attractive alternatives to meth acrylates due to low allergenic hazards low toxicity and fast polymerization.
Carboxylic acid pka 4 5 4.
Ammonium ion pka.
This would for example allow for fatty acids or other carboxylic acids to be used which can make the vinyl ether monomers more bio based 14 15 in this work we present a novel method that can be used to.
The reaction of saturated carboxylic acids with vinyl ethers gave the corresponding esters.